Introduction
The concept of ageing is a mysterious process and raises many questions. Why do organisms age? Why do we die at different ages? What are the mechanisms that underlie ageing? This is especially intriguing when scientists observe varying lifespans of one laboratory mice strain, consisting of genetically identical individuals. With ageing comes the prospect of increased cellular dysfunction, affecting normal tissue functions leading to the development of age-related diseases, such as cancer, neurodegenerative disorders, and cardiovascular diseases (CVD). The average life expectancy of humans has improved massively worldwide, partly due to better improvements in medical treatments, diagnosis, and the healthcare system. With humans living longer, this brings an increased burden on the healthcare system to take care of the elderly, who may be suffering age-associated deficits / problems, as well as the prospect of having an older working population, whose productivity may be lower. A common feature of the elderly is increased frailty and the development of dementia as they approach their eighties. Why is ageing associated with these deficits, and are there ways to circumvent these shortcomings and potentially live forever?
Most of our current understanding of ageing has relied upon studies performed in animal and single-cell models, such as laboratory mice, the nematode worm, Caenorhabditis elegans (C.elegans), and the yeast Saccharomyces cerevisiae (S.cerevisiae). Despite the huge number of ageing studies, our understanding of ageing is limited since these models only simulate some aspects of ageing features. Hence, we are still unable to answer the questions posed above. I wish to direct the reader to the concepts of "chronological" versus "biological" ageing. The former refers to the amount of time passed from birth to a given date, while the latter occurs when the organism gradually accrues irreversible damage (to macromolecules, such as DNA, proteins, lipids) within their cells. Here we will discuss biological ageing, in terms of some of the ageing traits and what we might do to slow the effects of ageing to live a long and fruitful life.
Signs of Ageing
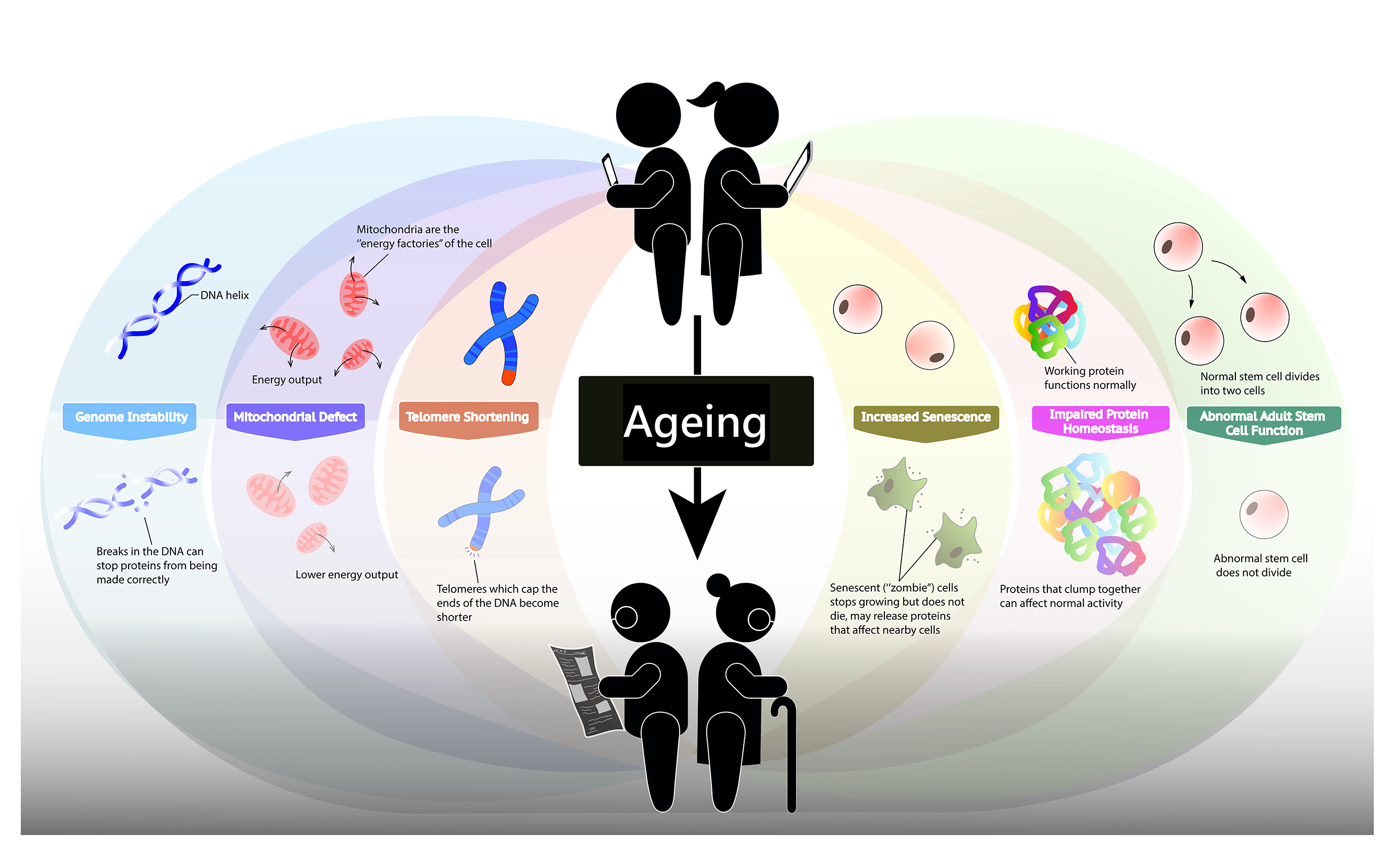
Genetic instability
Ageing is well-associated with the buildup of genetic damage throughout life. During cell division, the cellular genome is replicated by cellular machineries, but this is error-prone, leading to the generation of mutations and increased production of reactive oxidative species (ROS). Exposure to chemicals (e.g., ultraviolet radiation, UV) can also play a role in causing DNA damage. Depending on the type of DNA damage, cells can respond by activating cell cycle checkpoints, which pauses the cell cycle, giving the cell time to undergo DNA repair and activating pathways to break down proteins as needed. Cells have multiple DNA repair pathways key to preserving the integrity of the cellular genome and are considered to play an essential role in maintaining lifespan. Indeed, several genetic defects in DNA repair are associated with several premature ageing disorders.
Some premature ageing diseases occur because of accumulated DNA damage, but such diseases can only recapitulate some aspects of the ageing process. These genetic irregularities can vary, and their location can affect the function of important genes, leading to cellular dysfunction. Should these dysfunctional cells fail to be removed by apoptosis or senescence (refer to the section on senescence below), their presence can disturb tissue homeostasis and possibly lead to the development of diseases, such as cancers.
Changes in mitochondrial function
Mitochondria serve as the major organelle to produce energy (in the form of ATP) for the cell to carry out its daily functions. These organelles also contain their own DNA, passed down from the mother, and are a source of reactive oxygen species (ROS), a by-product of ATP synthesis. Mitochondrial DNA (mtDNA) is prone to ageing-associated mutations partly due to ROS release and the absence of histones that protect the DNA from damage. Furthermore, DNA repair pathways are also less efficient than those found in the nucleus. These mutations are more commonly found in aged cells and affect the respiratory chain complexes, which produce ATP in different tissues. The generation of mutant mice missing a mitochondrial enzyme (which replicates mitochondrial DNA) led to premature ageing and reduced lifespan. Deletions in the mtDNA were also found in the heart, brain, and muscle of ageing humans, providing strong evidence that maintaining mtDNA stability plays a key role in healthy ageing.
Over time, the efficacy of respiratory proteins leads to increased electron leakage and decreased ATP production. Dysfunctional mitochondria can suffer from changes in membrane stability and mitochondrial dynamics. Cells may remove defective mitochondria by mitophagy, where mitochondria are "eaten" by vesicles to break them down. Defects in mitophagy have been associated with Alzheimer's disease (AD) and other neurodegenerative disorders. Studies also found telomere maintenance contributed to mitophagy. Mice lacking the enzyme telomerase showed reduced numbers and growth of mitochondria, affecting proper mitochondrial function in cells. Subsequent telomere activation partially reversed this mitochondrial decline. Scientists suggested gradual mitochondrial dysfunction during ageing might increase ROS levels, which worsens the ability of organelles to function normally. However, scientists also noticed increasing ROS extended lifespan and that increasing antioxidant defenses failed to extend lifespan in animals challenging this theory. Nonetheless, mitochondrial dysfunction is a well-established sign of ageing.
Telomere length
As mentioned previously, increasing DNA damage correlates with age, with telomeres (located at the terminal ends of chromosomes) being highly vulnerable. DNA damage may lead to apoptosis or senescence. Replicative enzymes cannot completely replicate the telomere, which is only possible by telomerase activity. However, telomerase expression is absent in most mammalian cells, resulting in progressive telomere shortening. Furthermore, DNA repair is largely restricted at these sites as they are usually protein-bound to stop telomeres from being recognised as damaged DNA under normal circumstances. Defects in telomeres are associated with the early development of human diseases. Animal models lacking telomerase had decreased lifespan, but telomerase activation led to a longer lifespan. It should also be noted that a recent study of cancer-linked mutations found that having long telomeres appeared to increase the risk of cancer in humans. Telomere shortening, while associated with early ageing, also serves a role in preventing cancer development as it can stop cell division. Taken together, this suggests telomere length is not a reliable measurement of cellular age.
Senescence
Senescence is a state of irreversible cell cycle exit that may occur in response to cellular stress. Senescent cells can be defined using a group of proteins secreted by senescent cells termed "senescence-associated secretory phenotype." These secreted factors, such as growth factors, inflammatory cytokines, and enzymes, can affect neighbouring cell growth. These cells can be identified because they expressed increase levels of known proteins, seen in ageing-associated diseases like CVD and AD. Removing these proteins in premature ageing animal models (which showed signs of senescence) improved the state of the affected cells. As senescence increases during ageing, it was assumed to contribute to ageing. However, mice showing small increases of senescent-associated proteins showed extended lifespan. This observation suggested that the initial accumulation of these proteins might be a way for the cell to respond, but excessive senescence activation instead worsens cellular functions.
Problems in protein homeostasis
Ageing is well-established to be linked to impaired protein homeostasis. Homeostasis refers to the ability to maintain a stable environment by the cell having a range of quality control (QC) pathways to ensure proper folding of proteins and adequate removal of dysfunctional proteins. Overexpression of these QC-related proteins in worms and flies led to increased lifespan. For a protein to function normally, it must be folded correctly and may under severe conditions leading to protein aggregation. Misfolded proteins have been linked to ageing-related disorders such as AD and Parkinson's disease.
The autophagy-lysosomal pathway is also important in maintaining protein homeostasis. This process of "self-eating" allows dysfunctional or unnecessary cellular components to be removed from the cell by fusing targets with a vesicle containing digestive enzymes to degrade the contents. However, this decreases gradually with age, which has been linked to neurodegenerative disorders and cancer. Autophagy can be increased using drugs, which leads to extended lifespan in yeast and nematode worms and delayed some signs of ageing in mice. In summary, loss of protein homeostasis is a common feature in ageing-related disorders and may be a target for future anti-ageing therapies.
Adult stem cell function
Adult stem cells (ASCs) are important to maintaining tissue functions and overall health. They reside in specific tissues, such as the intestine, bone marrow, liver, and skeletal muscle. These stem cells generally take part in tissue maintenance and restoration. A subset of these stem cells resides in the quiescent state (separate from the cell cycle), whereby they can quickly respond to a stimulus (such as tissue injury), activate, and re-enter the cell cycle. One stem cell divides into two daughter cells, where one daughter cell will be destined to differentiate into one of many cell types that make up that tissue, while the other returns to quiescence as a stem cell. This mechanism can protect ASCs from collating DNA damage and maintains the resident stem cell pool. However, as we age, ASCs begin to lose their ability to activate, reducing the ability of the tissue to regenerate. ASCs may display abnormal cell division and failure to return to the quiescent state, depleting the stem cell pool, leading to reduced stem cell numbers in older organisms compared to young in some situations.
A subset of stem cells in the bone marrow, liver, and spleen are called hematopoietic stem cells (HSCs). HSCs are one of the well-studied ASCs. They allow for the generation of blood cells (including red blood cells and immune cells) in a process called hematopoiesis. This process declines with age, leading to reduced numbers of immune cells and blood cells, resulting in a weakened immune system (and therefore reduced ability to combat infection), increased incidence of anemia, and increased risk of developing cancers. HSCs in aged rodent models show decreased cell-cycle activity and reduced regenerative capacity.
Mutations can collate in stem cells. Research investigating leukemia-related mutations unveiled that normal blood cells commonly carry more mutations unrelated to leukemia than expected. Blood cells were largely thought to be derived from 1000 active stem cells in young adults, but many of these blood cells carried the same mutation, suggesting a single stem cell is responsible for those blood cells in some individuals. This is increasingly seen in older people, which correlates with an increased risk of CVD, stroke, and diabetes. Several mutated genes were identified in these stem cells, commonly genes that encode proteins that control gene expression. Mice modelling different blood disorders commonly had mutations in stem cells, which led to a loss of stem cell maintenance. A gene that under normal conditions regulates responses to stress and DNA damage is commonly mutated in cancers but also controls HSC cell division. HSCs missing this gene suffered from reduced cell division and reduced ability to regenerate tissue when transplanted into other organisms.
The skeletal muscle consists of muscle stem cells (also called satellite cells (SCs)) located under the basal lamina of muscle fibers. SCs play a major role in muscle regeneration after injury. However, the regenerative process in aged muscles is reduced due to the reduced capacity of stem cell division, thereby slower differentiation of stem cells into new myofibers. SCs are also sensitive to their surrounding environment, affecting their ability to function properly. Changes in ageing have been known to alter the microenvironment, which can lead to muscle loss. In human ageing, loss of muscle mass and strength is known as sarcopenia, increasing the individual's risk of falls, physical disability, and death. The biology of sarcopenia is unclear, but generally, multiple factors such as activation of inflammation, abnormal mitochondrial function, loss of muscular structures, and reduction in stem cell numbers are all thought to be involved.

Fig 1: Signs of ageing
Mysteries of the ageing process
The ageing process is generally accepted as a consequence of all organisms, with increasing age linked with increased mortality. The ability of an organism to ensure healthy function during adult life depends on homeostasis, which must be tightly regulated; otherwise, this may lead to cellular dysfunction and, therefore, the development of disease. However, several key experiments have changed how we understand ageing. Parabiosis describes the process by which two organisms (one young, the other old) are joined surgically so that age-related and disease-related changes in the blood can be investigated. Aged stem cells exposed to a young environment showed more youthful traits, which means it might be possible to restore certain ageing features. Young stem cells exposed to an ageing environment demonstrated ageing-related defects in cellular functions, showing that stem cells are highly influenced by their environment and that factors released in the blood may be the key to reversing ageing.
Several ageing-associated blood factors from the parabiosis experiments have been identified as either inflammation-related or immune-system-related proteins. Examples are cytokines which affect neuronal cell growth and division. Increased levels of other proteins were found with increasing age and even affected muscle tissue regeneration. Taken together, this demonstrates that inflammation plays a role in the ageing process.
Experiments to understand how ageing could be reversed came from studying the process of fertilisation of the ovum by sperm, where two cells come together to form a single cell that removes any traces of the parental cellular ages. Early experiments where the nucleus was taken from tadpole muscle and transferred into nuclei-depleted Xenopus eggs led to healthy male and female frogs, showing that the nucleus was somehow reprogrammed by the environment of the ovum. Studies of the molecular signatures of young and old cells were revealed. In contrast, all cells carry identical genomic DNA, and gene transcription is regulated by epigenetics, enabling genetically identical cells to become different cell types.

Fig 2: Epigenetics
Epigenetics refers to changes in gene expression not encoded by the DNA through chemical changes of chromatin, the structure of DNA, RNA, and protein. DNA consists of a long chain of single units of nucleotides, which can be chemically modified. Two chemical changes are well characterised; the methylation of cytosines (found on some nucleotides) and acetylation of lysines (at histones) carried out by specific enzymes to alter gene expression. Increased histone acetylation or trimethylation at particular genome locations make up what we call age-associated epigenetics marks, whereas removing these marks during parabiosis may allow aged cells to be rejuvenated to that of young, healthy cells.
There is a great interest in knowing which cellular pathways lead to dysregulated control of gene expression, contributing to declines in stem cell function during ageing. Histone methylation is associated with ageing in invertebrate models, where the loss of a gene that leads to increased methylation leads to a phenotype with an extended lifespan. A specific marker of histone methylation associated with promoters driving gene expression is linked with reduced lifespan. Conversely, yeast expressing reduced levels of another histone mark demonstrated reduced longevity. DNA methylation was used as an ageing biomarker to estimate the age of tissues across the entire human lifespan. Furthermore, studies comparing centenarians (humans who have reached the age of 100) and middle-aged persons showed changes in methylated genes, some of which were disease-related.
What can we do to protect against ageing?
There are currently no available anti-ageing drugs on the market. However, studies of environmental factors and their benefits to improving cellular health are well supported. Calorie restriction (CR) has been proven to increase longevity in animal models, in part due to enhanced autophagy and increased metabolism of fats, leading to weight loss. However, CR is somewhat difficult to maintain in humans, so nutritionists came up with intermittent fasting (IF), where the individual eats within an 8-hour period but fasts for the remaining 16 hours of the day. IF also improved autophagy, glucose tolerance, and a metabolic switch from glucose to ketone-based energy sources.
Interestingly, CR reduced age-related changes in DNA methylation in Rhesus monkeys. CR and IF have been suggested to be useful interventions for those who are pre-diabetic, as well as for combating obesity. The benefits of keeping to certain diets were observed using the known example of the Mediterranean Diet, which has long been established to be linked to longevity and protection from CVD and cancer. The diet is high in vegetables / plant-based foods, fruits, fish, and olive oil, consistent with increased amounts of vitamins, antioxidants, and omega-3 fatty acids, contributing to better cellular health.
Exercise or physical activity is highly beneficial for human health, such as improving cardiovascular health and strengthening skeletal muscle. It has also shown promising results in clinical studies and experimental models of obesity. Mice undergoing cardio activity demonstrated increased autophagy in tissues, including the skeletal muscle, heart, and liver. Long-term exercise also improved high fat-induced glucose intolerance in mice. The benefits of exercise on stem cell function were demonstrated in mice provided with a running wheel. In this study, running accelerated muscle repair in older mice and improved aged SC function compared to young stem cells. Exercise also partially revitalised transcriptional changes in ageing and alleviated some signs of cellular stress in old muscle stem cells. Resistance exercises can strengthen muscle mass and function, with its effects found to have the same benefits seen in younger adults in patients with sarcopenia, highlighting that loss of muscle mass can be restored through external means.
Other advantages of exercise include increased mitochondrial biogenesis, enhancing ATP production in skeletal muscle, and increased mitophagy. Apart from the cardiovascular benefits of aerobic exercise, exercise also promotes neuronal health partly through increased secretion of growth factors to support the survival of existing and new neurons and processes that support learning and memory. A trial involving people with mild cognitive impairment who performed aerobic exercise showed reduced hippocampus shrinkage, improved brain functions, and better memory.
Concluding remarks
The elderly make up an increasing proportion of the human population, especially in more developed countries with better access to healthcare and social services. The major causes of human mortality are largely due to diseases occurring in the elderly, who tend to be less able to combat infection and suffer from poorer mental and physical health. Yet, there are individuals who lead active lives, living well into their hundreds. Do the secrets of delayed ageing lie in these select few? What we do know is that environmental factors, like lifestyle, can influence the cellular capacity to function normally and possibly to some extent at the genetic level, to slow the impacts of ageing. However, there is still much we do not know about the biology of ageing. This has driven scientists to understand ageing better and, ultimately, develop therapies that could improve the quality of health and lifespan as humans become older.
Glossary of Terms
Cancer: a group of diseases involving uncontrolled cell growth, forming tumours.
Neurodegenerative disorders: a group of diseases involving death or abnormal function of nerve cells.
Cardiovascular diseases (CVD): groups of diseases involving abnormal functions of the heart or blood vessels.
Frailty: physical condition of weakness, common in ageing.
Dementia: general term loss of memory or other brain functions interfering with daily life.
Mutation(s): changes in the DNA sequence.
Reactive oxygen species (ROS): unstable oxygen-containing molecules that can react with DNA, RNA, or proteins to cause damage.
Apoptosis: an ordered process leading to cell death, describes how tissues remove cancerous or dysfunctional cells.
Senescence: an irreversible state where a cell begins to decline in function and stop dividing.
ATP: adenosine triphosphate, an energy-carrying molecule used by cells to perform daily functions.
Histones: proteins wrapped by DNA, which usually protect DNA from damage.
Alzheimer's disease (AD): an irreversible brain disease due to death or abnormal function of neurons affecting memory and thinking abilities.
Mitophagy: a process of removing damaged mitochondria from a cell by degradation.
Vesicle: a structure inside / outside a cell that carries cellular material.
Antioxidant: chemicals that counteract ROS, also known as "free-radical scavengers," are generally protective.
Telomerase: an enzyme that maintains telomeres.
Telomere: found on the ends of DNA, protecting the DNA from fusing to other DNA.
Parkinson's disease (PD): brain disease, due to death or abnormal function of neurons, leading to shaking, stiffness, and difficulties in movement.
Autophagy: the natural process of a cell removing unneeded or dysfunctional components, also known as "self-eating."
Differentiate: when a cell changes from one type into another.
Quiescence: a reversible state where a cell does not divide unless needed, seen in adult stem cells (it is not dormant).
Leukemia: a type of white blood cell cancer.
Stroke: poor blood flow to the brain causing cell death.
Diabetes: a disease where glucose blood levels stay high, causing glucose to build up in the blood.
Sarcopenia: slow loss of skeletal mass and strength seen in the elderly.
Parabiosis: laboratory method where two organisms are linked to a share physiological system (e.g., fascia, blood supply).
Inflammation: biological response of body tissues to harmful stimuli (e.g., infection, tissue damage).
Cytokines: a broad group of proteins released by cells to signal other cells during inflammation.
Epigenetics: the study of changes in gene expression, not encoded in DNA.
Methylation: the chemical process whereby -CH3 groups are added to a compound. When added to DNA or histones, it controls gene expression.
Acetylation: the chemical process whereby -OOH groups are added to a compound. When added to histones, it controls gene expression.
Lysine: basic amino acid, a building block to make protein.
Nucleotide: building block that makes up DNA and RNA.
Cytosine: one of the four chemical bases that make up RNA and DNA.
Biomarker: measurable variable that may suggest signs of disease.
Calorie restriction (CR): decreasing food intake, below daily calorie intake.
Ketone: molecules released after the breakdown of fatty acids, may occur during fasting or after intense exercise.
Mild cognitive impairment (MCI): a general brain condition describing the steady loss of thinking abilities, increases the risk of developing AD later in life.
Author profile
Prof Tom Cheung is currently the S H Ho Associate Professor of Life Science in the Division of Life Science at The Hong Kong University of Science and Technology (HKUST). He received his PhD in Biochemistry from the University of Colorado, United States of America. He specialises in the field of stem cells and the biology of ageing using murine muscle stem cells to identify the key molecular pathways that underlie stem cell quiescence and tissue regeneration. Prof Cheung is also a recipient of the Croucher Innovation Award in 2015 for the study of "Molecular regulation of stem cell ageing" and has published more than 30 scientific papers in high impact journals. Furthermore, Prof Cheung is Director of the HKUST-Nan Fung Life Sciences Joint Laboratory, Associate Director of the Biosciences Central Research Facility at HKUST, Director of the HKUST-BGI Joint Research Center, and Associate Director of the HKUST-Shanghai Sixth People's Hospital Joint Research Center for Brain Science, as well as being a key member of the Hong Kong Center for Neurodegenerative Diseases (HKCeND) at the Hong Kong Science Park.
Dr Erin Tse received her PhD in Biomedical Sciences from Aston University, United Kingdom, studying Alzheimer's disease under the supervision of Dr Eric Hill. To further her personal growth, Erin moved to Hong Kong working in microbiology, followed by some time in a virology group using human stem-cell-derived enteroids to study the host interactome of human norovirus structural proteins. Through her experiences, she specialises in the study of cell biology. Currently, Dr Tse is a Postdoctoral Fellow in the Cheung Laboratory, where she continues to further stem cell research. Dr Tse is also an active member of the HKUST-Nan Fung Joint Sciences Laboratory, largely responsible for manageing the joint lab activities and mentorship of trainees.
March 2022