Background: Global warming continues to pose a threat to human society and the ecological systems, and carbon dioxide accounts for the largest proportion of the greenhouse gases that dominate climate warming. To combat climate change and move towards the goal of carbon neutrality, researchers from The Hong Kong Polytechnic University have developed a durable, highly selective and energy-efficient carbon dioxide (CO2) electroreduction system that can convert CO2 into ethylene for industrial purposes to provide an effective solution for reducing CO2 emissions. This research was recently published in Nature Energy and won a Gold Medal at the 48th International Exhibition of Inventions Geneva in Switzerland.
Ethylene (C2H4) is one of the most in-demand chemicals globally and is mainly used in the manufacture of polymers such as polyethylene, which, in turn, can be used to make plastics and chemical fibres commonly used in daily life. However, it is still mostly obtained from petrochemical sources and the production process involves the creation of a very significant carbon footprint.
Durable Electrocatalytic CO2 Reduction to Ethylene System via Eliminating Carbonate Formation
Introduction: In the fight against climate change, finding efficient ways to reduce CO2 emissions is crucial. Electrocatalytic CO2 reduction (ECO2R) using renewable energy sources has emerged as a promising solution [1,2]. However, the presence of alkaline conditions and alkali cations during ECO2R poses challenges, hindering the development of this technology [3,4]. We explore a groundbreaking pure-water-fed membrane-electrode-assembly (MEA) system that overcomes these obstacles, paving the way for a more stable and efficient ECO2R process.
Efficient CO2 reduction is crucial in fighting climate change. ECO2R with renewable energy is promising, but alkaline conditions and alkali cations pose challenges. Our pure-water-fed MEA system overcomes these, paving the way for a stable and efficient ECO2R process.
The Pure-water-Fed MEA System: The pure-water-fed membrane-electrode-assembly (MEA) system consists of an anion-exchange membrane (AEM) and a proton-exchange membrane (PEM) assembly (APMA, Fig 1). This innovative system maintains an alkaline cathode environment necessary for effective ECO2R. Under forward bias, water dissociation occurs at the cathode and anode, participating in ECO2R and oxygen evolution reaction (OER). The remaining OH- and H+ ions are transported through AEM and PEM, respectively, forming water at their interface.
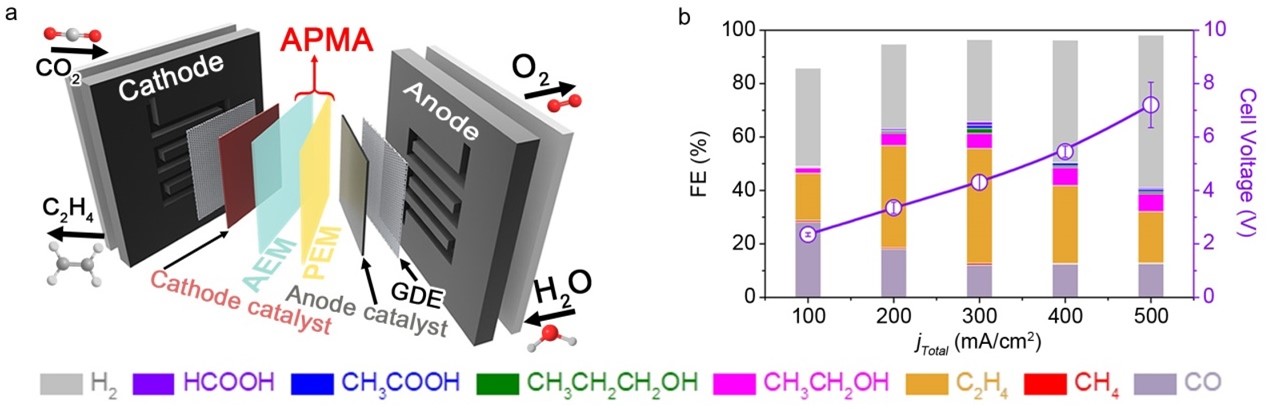
Fig 1: (a) Schematic of the pure-water-fed APMA-MEA system architecture for ECO2R. (b) ECO2R performance on SS-Cu in the pure-H2O-fed APMA-MEA cell, and the corresponding cell voltages without iR compensation.
Results: Using a high-performance surface-step-rich Cu (SS-Cu), the ECO2R product distribution in the pure-water-fed APMA-MEA system was examined (Fig 1b). The results were remarkable, with a peak Faradaic efficiency (FE) of approximately 66%, including a significant 43% FE towards C2H4 (ethylene). The cell voltage was around 4.3 V, and the full-cell energy efficiency reached up to 18.2% for ECO2R.
Preventing Carbonate Formation: One of the major challenges in ECO2R is the formation of carbonates and salt precipitation. However, the pure-water-fed APMA-MEA system architecture drastically reduces the probability of these reactions. The absence of cations, under the influence of the electrostatic field, effectively suppresses (bi)carbonate salt formation and prevents salt precipitation.
Experimental Evidence: In-situ Raman measurements directly demonstrated the effective suppression of carbonate formation on the SS-Cu surface in the pure-H2O-fed APMA system (Fig 2a). Additionally, an isotope labeling experiment using H218O as the anolyte confirmed that CO2 did not react with the electrogenerated OH- into carbonate (Fig 2b). These findings provide strong evidence that the pure-water-fed APMA-MEA system configuration successfully prevents carbonate formation and anion crossover.
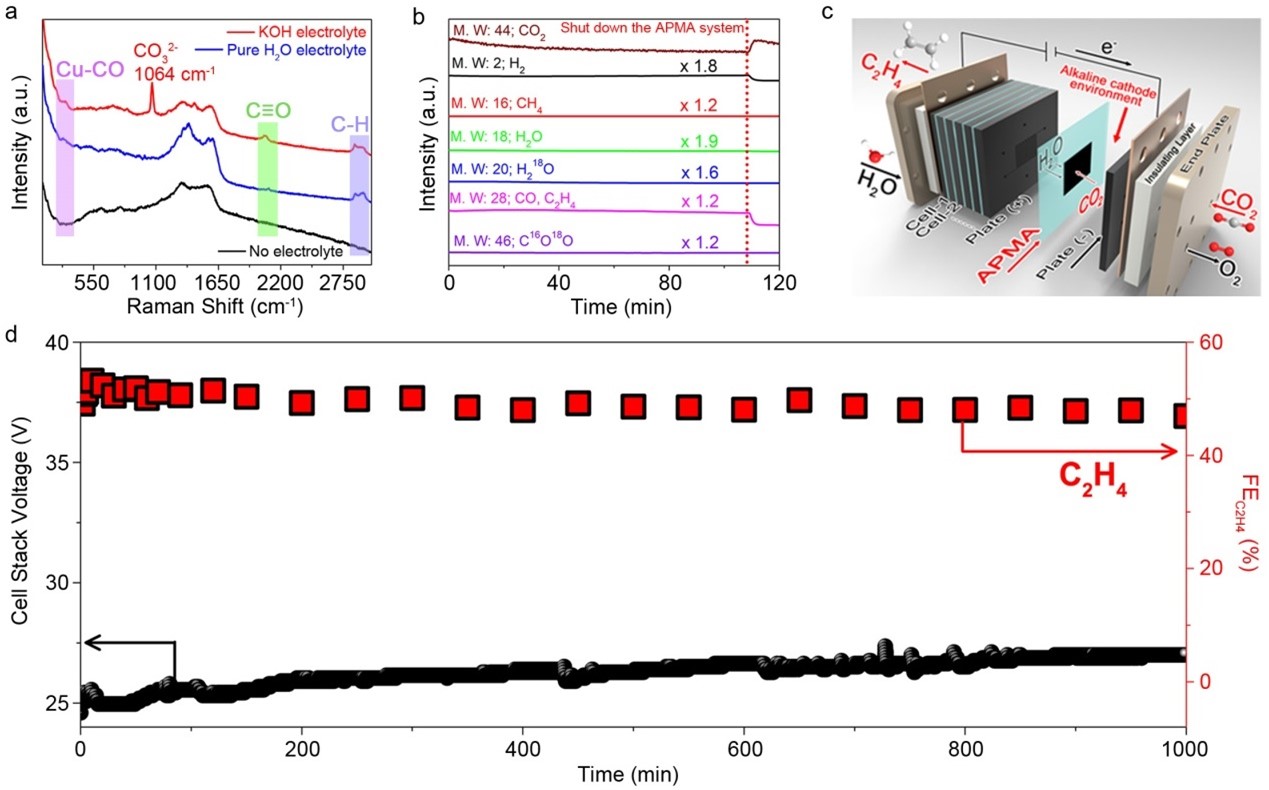
Fig 2: (a) In-situ Raman spectra of ECO2R on SS-Cu in 0.1 M KOH, pure H2O and bare electrode after ~20 min of ECO2R. (b) Mass spectra of ECO2R using H218O as the anolyte in the APMA system. (c) System stability performance of ECO2R-to-C2H4 on SS-Cu in a pure-H2O-fed APMA-MEA cell stack containing 6 APMA-MEA cells at a constant current of 10 A.
Durability and Practicality: To evaluate the durability and practicality of the pure-water-fed APMA-MEA architecture, a cell stack containing six MEA cells was designed (Fig 2c). The results were highly promising, with a ~50% FE towards C2H4 achieved at a total current of 10 A (Fig 2d). The system remained stable for over 1000 hours, demonstrating its long-term viability and potential for industrial-scale implementation.
Conclusion: The pure-water-fed MEA system represents a significant breakthrough in the field of CO2 reduction. By effectively suppressing carbonate formation and salt precipitation, this innovative technology offers a more stable and efficient approach to ECO2R. With its potential to bridge the gap between fossil fuels and sustainable energy, this system brings us one step closer to a greener and more sustainable future.
References:
- De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
- Wakerley, D. et al. Gas diffusion electrodes, reactor designs and key metrics of low-temperature CO2 electrolysers. Nat. Energy 7, 130-143 (2022).
- Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
- She, X. J., Wang, Y. F., Xu, H., Tsang, S. C. E. & Lau, S. P. Challenges and opportunities of electrocatalytic CO2 reduction to chemicals and fuels. Angew. Chem. Int. Ed. 134, e202211396 (2022).
Author:
Prof Daniel Lau, Chair Professor of Nanomaterials and Head of the Department of Applied Physics, The Hong Kong Polytechnic University
March 2024