A dog's nose is regarded as one of the most powerful sensors that exist. Trained canines are even able to detect various clinical conditions, such as spikes in blood sugar and cholesterol levels, by sniffing the affected person. For routine clinical examinations, we would need a more practical and cost-effective way to "sniff". For my PhD project, my research was centered on developing a new method of integrating a 'dog's nose'-like system in our everyday device, using a material called metal-organic frameworks.
Fig 1: Alex in the chemistry lab and inside the nanoengineering cleanroom – research that traverses various subject boundaries: materials science, chemistry, and nanodevice technology
The most commonly known electronic nose is the breathalyser. As drivers subjected to these tests breathe into the equipment, a sensor embedded onto the breathalyser measures the amount of alcohol in their system by correlating the concentration in their breath. The chemical interaction of the alcohol molecules and the active sensor material in the breathalyser is then transformed into an electronic signal, allowing the investigating officer to read off the result. Alcohol in our breath is relatively easy to detect because the chemistry is specific, and the alcohol concentration is fairly high (even without having an excess drink or two). But our breath is comprised of complex mixtures of gas molecules in very low concentrations. And fabricating state-of-the-art electronic noses to detect specific molecules at lower concentrations (ppm-level, about a drop of water in an Olympic-sized swimming pool) is a challenge. Fortunately, there is a solution for this limitation which is enabled by chemistry and materials science. Very sensitive electronic noses of the future can now be fabricated with a material called metal-organic frameworks (MOFs).
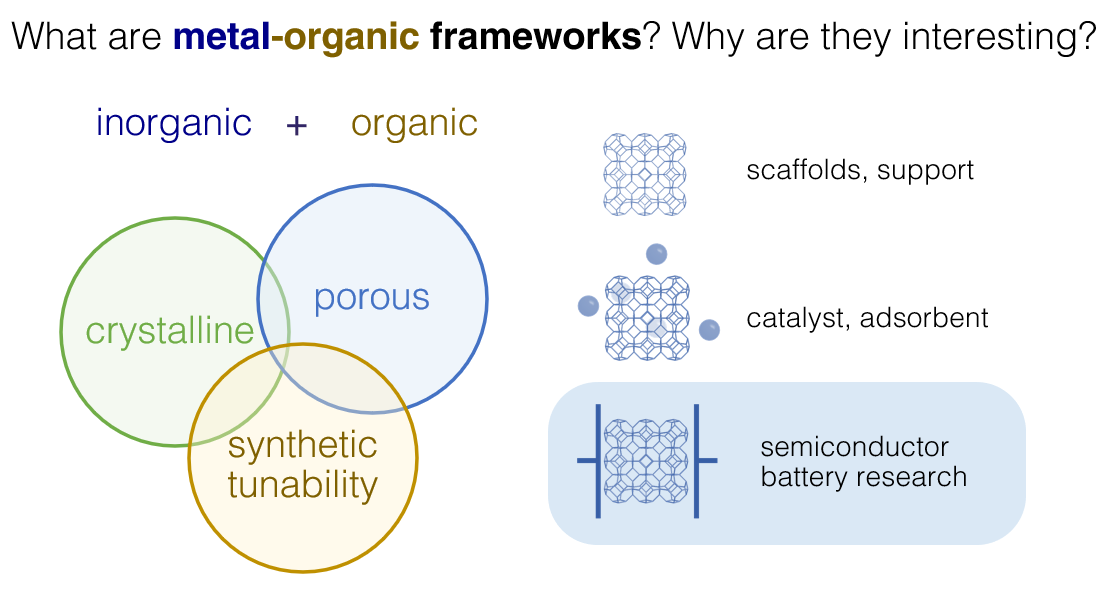
Fig 2: What are metal-organic frameworks, and what makes them so interesting?
Porous materials have garnered tremendous interest within the scientific community and the industry attributed to their gas sorption performances (sensors and gas storage), capabilities enabling chemical processes (catalysis for chemicals production), energy storage capacities (batteries and their components), and many other captivating applications. MOFs are a subclass of these porous materials and are very tiny, porous sponges in the nanoscale (dimension of about 100,000 times smaller than a diameter of a human hair). They have unique properties suitable for high-value applications such as integration into microelectronic devices. Moreover, this 'sponge-like' behaviour at the nanoscale also provides opportunities to be used as high-performance isolators to develop faster microprocessors for the next generation of supercomputers.
How can this be used as a dog's nose?
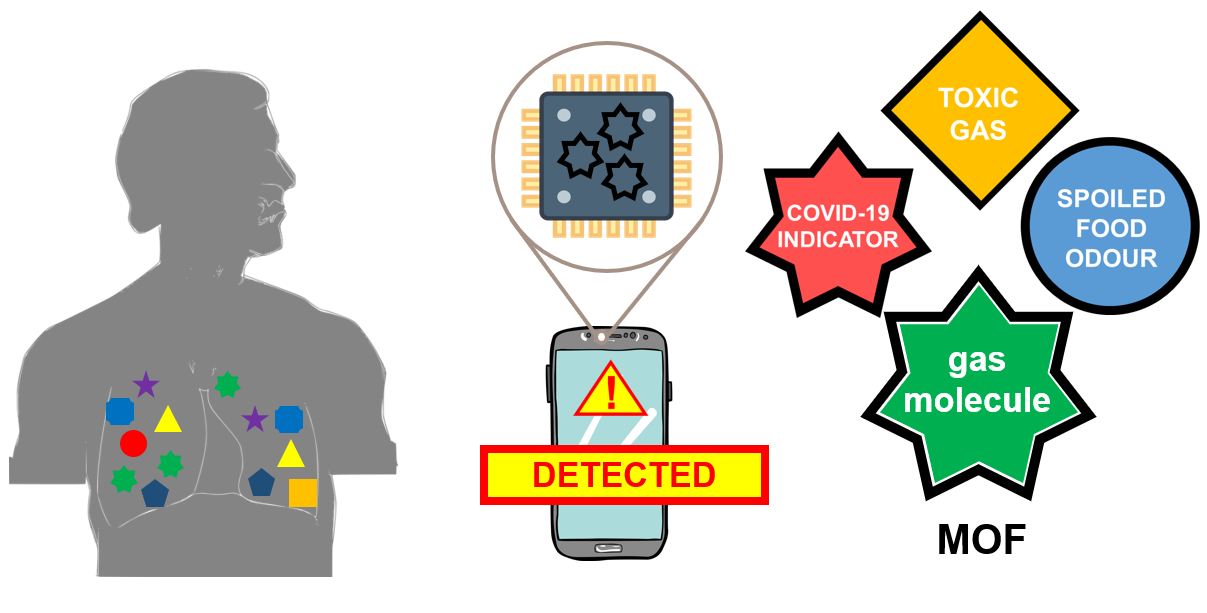
Your breath comprises many types of gas molecules that may be used to infer your health condition. Specific gas molecules associated with certain types of diseases, such as lung cancer, are very difficult to detect using normal sensors. As these gas molecules assume certain shapes and sizes down at the molecular level, we can tune the shape of a MOF for a certain gas molecule, and it will act as your super-sensitive sensor as it is integrated into an electronic device. The shapes and sizes of these MOFs can be designed to distinguish a target molecule you want to detect, such as markers of diseases traced in human breath, toxic gases, and even odours coming from spoiled food. The levels of certain molecules in your breath will give you and your doctors an indication of whether it's time for a more detailed check-up on your lungs. These sensors may be repurposed to check the quality of air that you breathe in your home or workplace. Or check whether the leftover food you stored in the fridge for three days is still good for dinner.
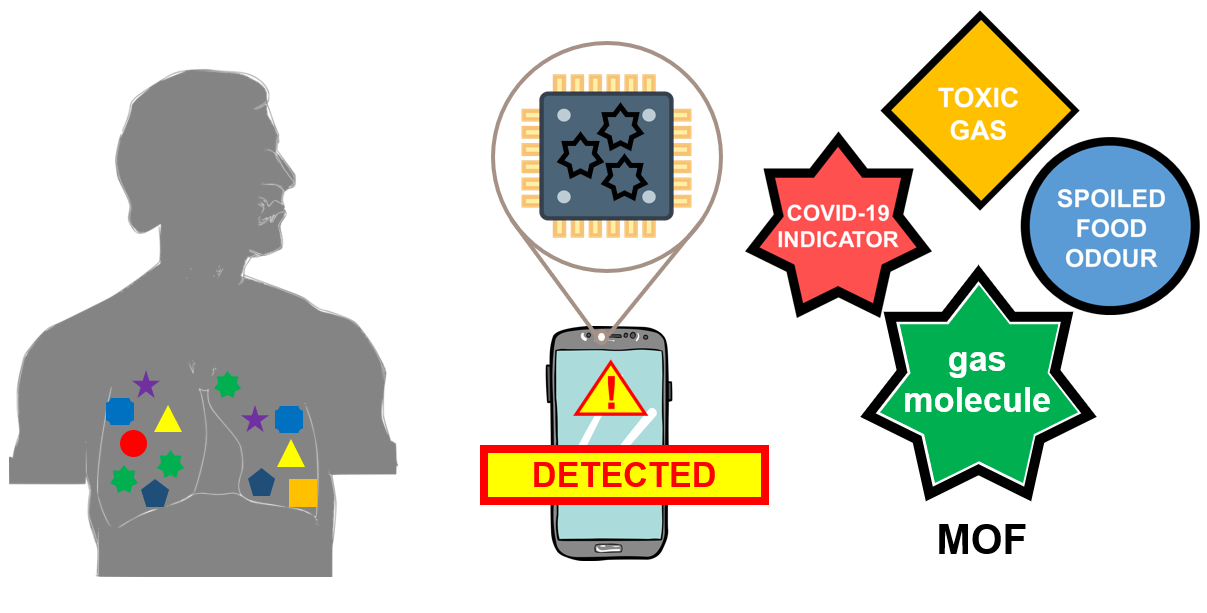
Fig 3: How can these be integrated into your smartphones?
In order to valorise the industry-scale integration of MOFs on electronic devices, the manufacturing process of these porous materials for these applications needs to comply with a set of stringent qualifications:
- MOF materials can be deposited as very thin films; and
- It can be processed in very little or without any solvent
These conditions are key technological bottlenecks for the porous materials, such as MOFs, that have been tested so far in this context. In my PhD work, we employed the creation of these microporous coatings to address the two challenges above – thin film deposition of MOFs in the absence of solvents – a recently developed technique in our lab for processing these types of materials.
While solution-based MOF synthesis, typically via powder preparation routes, carries advantages for its simplicity and cost-effectiveness, its fabrication as coatings compatible with production facilities is imperative for its device integration viability. Several challenges remain in traditional protocols, such as corrosion, contamination, and lack of control over the fabrication process. Furthermore, sustainable routes to generate functional materials have been one of the pillars of green industries and environmentally-friendly industrial processes. My research envisaged eliminating fabrication-related obstacles that hamper the commercial integration of MOFs in electronic devices via a solvent-free, industry-grade, and scalable cleanroom-compatible route. Here, chemical vapour deposition (CVD), a cornerstone in microfabrication, is deployed for MOFs.
CVD is a staple manufacturing methodology – a process applied in practically all materials from solar cells to your reading glasses – and is one of the critical steps in semiconductor manufacturing needed to produce state-of-the-art electronics. Expanding the CVD method for these sponge-like MOFs will result in high-performance materials ready to meet increasingly demanding specifications in solid-state devices, i.e., extending Moore's law. In an archetypal CVD process, a substrate (typically a silicon wafer) is exposed to a number of volatile precursors. These precursors will react on the substrate's surface to deposit the thin film with the desired thickness and properties. This protocol can be a continuous or batch process, characteristically coating tens or even hundreds of wafers in one exposure run. In the process so developed, we produced a MOF thin film – in the absence of solvents - via a slightly-modified CVD process. Instead, the new approach which consists of a precursor deposition step (typically a metal oxide) followed by a vapour exposure to the next precursor (organic linker volatilised at a certain temperature). This approach enables the deposition of thin films of MOFs with a consistent thickness that can be controlled and fine-tuned, even on complex geometric configurations (e.g., high-aspect-ratio features). Furthermore, this deposition technology can be applied in a large-scale reactor compatible with cleanroom fabrication standards. The demonstrated scalability of this process with existing microfabrication, both in academic and large-scale production sites, has proven to facilitate the integration of MOFs for device-related applications.
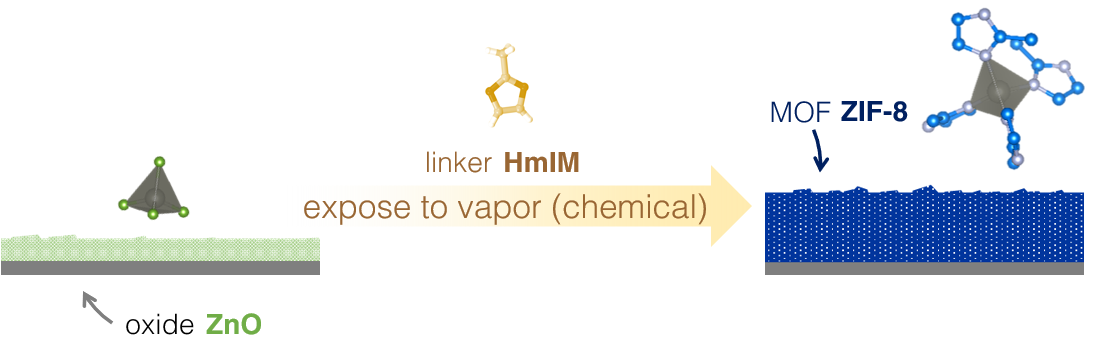
Fig 4: Chemical vapour deposition
Reference: Cruz, A. J., Stassen, I. Krishtab, M., Marcoen, K., Stassin, T., Rodríguez-Hermida, S., Teyssandier, J., Pletincx, S., Verbeke, R., Rubio-Giménez, V., Tatay, S., Martí-Gastaldo, C., Meersschaut, J., Vereecken, P. M., De Feyter, S., Hauffman, T., and Ameloot, R., Integrated cleanroom process for the vapor-phase deposition of large-area zeolitic imidazolate framework thin films. Chemistry of Materials 31 (22), 9462−9471 (2019)
On a broader scale, this newly-developed process would fully capitalise on the colossal potential industrial applications of MOFs (e.g., digital, photovoltaics, data storage, sensors). The research project paved the first steps to scale up this technology which could be readily adapted by industrial-scale facilities and test the performance of these coatings for high-value industries. This technology marks a significant milestone in opening doors for new and cutting-edge applications for commercial valorisation.
With CVD, we have developed methods to integrate these MOF materials into the electronics of everyday smartphone in a cheap, efficient, and scalable way. So that one day, these MOFs in a smartphone can be used not only in detecting drugs in airports, like a dog's nose, but also in the early diagnosis of certain types of diseases—a promising potential to revolutionise the healthcare sector.
References:
- https://www.sciencefiguredout.be/dogs-nose-your-smartphone
- https://amelootgroup.org/
- Cruz, A. J., Arnauts, G., Obst, M., Kravchenko, D., Vereecken, P. M., De Feyter, S., Stassen, I., Hauffman, T., and Ameloot, R., Effect of different oxide and hybrid precursors on MOF-CVD of ZIF-8 films. Dalton Transactions 50, 6784-6788 (2021) Link to Publisher
- Cruz, A. J., Stassen, I. Krishtab, M., Marcoen, K., Stassin, T., Rodríguez-Hermida, S., Teyssandier, J., Pletincx, S., Verbeke, R., Rubio-Giménez, V., Tatay, S., Martí-Gastaldo, C., Meersschaut, J., Vereecken, P. M., De Feyter, S., Hauffman, T., and Ameloot, R., Integrated cleanroom process for the vapor-phase deposition of large-area zeolitic imidazolate framework thin films. Chemistry of Materials 31 (22), 9462−9471 (2019). Link to Publisher
- Krishtab, M., Stassen, I., Stassin, T., Cruz, A. J., Okudur, O. O., Armini, S., Wilson, C., De Gendt, S., and Ameloot, R., Vapor-deposited zeolitic imidazolate frameworks as gap-filling ultra-low-k dielectrics. Nature Communications 10, 3729 (2019).
- Smets, J., Cruz, A. J., Rubio-Giménez, V., Tietze, M. L., Kravchenko, D. E., Arnauts, G., Matavž, A., Wauteraerts, N., Tu, M., Marcoen, K., Imaz, I., Maspoch, D., Korytov, M., Vereecken, P. M., De Feyter, S., Hauffman, T., and, Ameloot, R., Molecular Layer Deposition of Zeolitic Imidazolate Framework-8 Films. Chemistry of Materials, (2023). Link to Publisher
Author: Dr Alexander John Cruz, Technology Integration Leader - Climate Technology Solutions, Baker Hughes
Alex is the Engineering and Technology Integration Leader for Climate Technology Solutions at Baker Hughes. He oversees the segment's R&D, engineering strategy, capability development, and innovation. In 2022 alone, Alex received the prestigious International Union of Pure and Applied Chemistry (IUPAC) and Solvay Prize for Outstanding Scientists and the ALLY Energy ESG and Climate Champion Award. He sits in various industry groups in the energy transition space, notably on the Executive Committee of the International Energy Agency Greenhouse Gas R&D Program. He holds a double PhD in Bioscience Engineering and Engineering Sciences.
June 2023