When we think of alpacas, the first things that pop up in our minds may be holiday farms or alpaca fleece. However, if you ask a biomedical scientist, they may have thought of nanobody, fragment of a special type of antibody.
To appreciate why nanobodies are so special, we must first understand what an antibody looks like (Fig 1). A conventional antibody is composed of two heavy chains and two light chains. These chains are joined by disulfide bonds to form a Y-shaped molecule. The two tips of the Y-shaped antibody are called variable regions, which are responsible for binding to a target antigen. Variable regions of antibodies literally vary greatly and they determine what antigen an antibody will bind to. In addition to conventional antibodies, members of the camelid family, such as alpacas, camels and llamas, also produce a special type of antibody that only consists of two heavy chains [1, 2]. Nanobodies are the variable regions of these special antibodies.
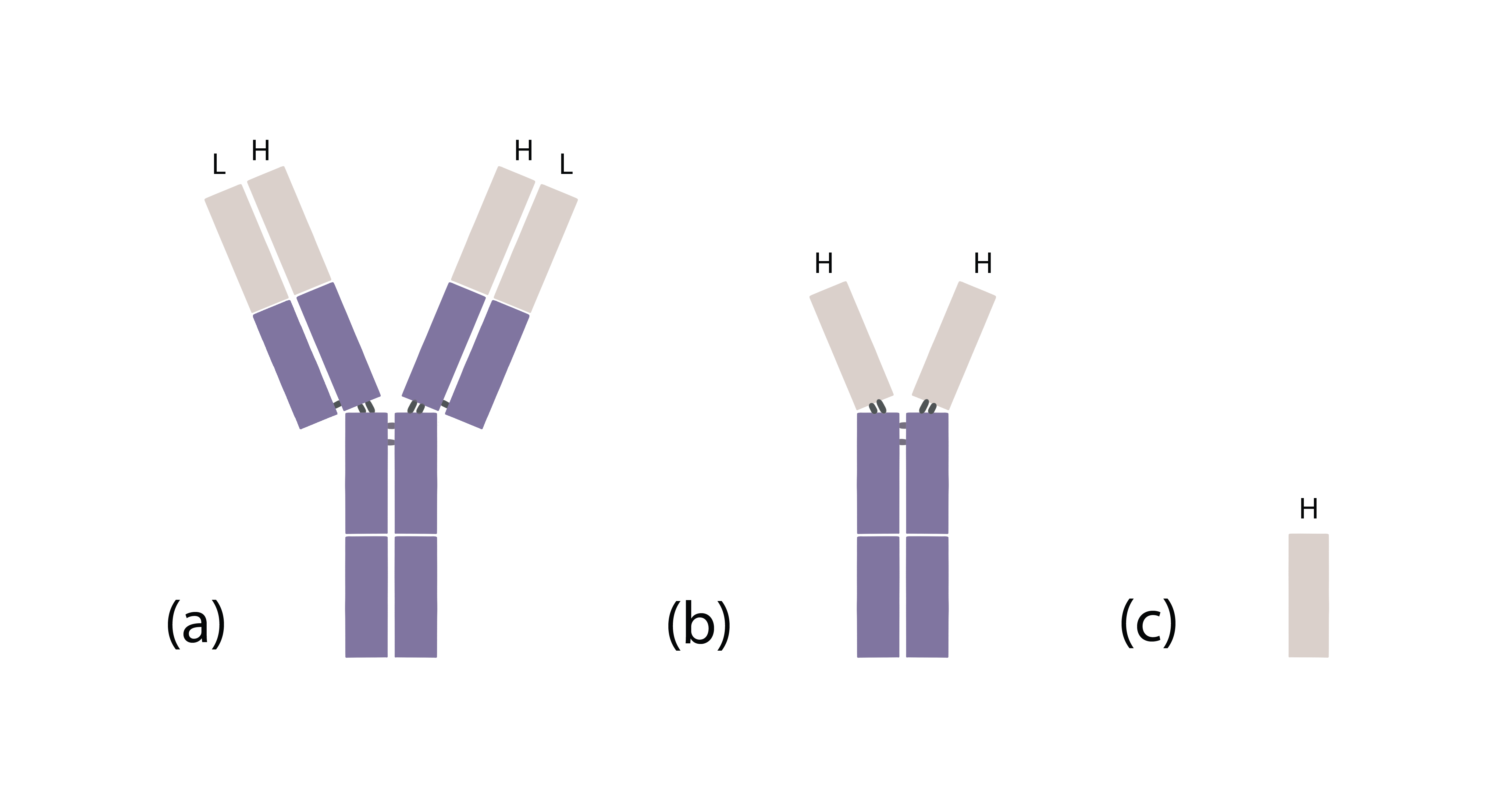
Fig 1: Structures of (a) a conventional antibody, (b) a camelid heavy chain-only antibody, and (c) a nanobody. (L: light chain, H: heavy chain; gray: variable region, purple: constant region)
It did not take long for scientists to notice the great potential of nanobody after it was first discovered in 1993 [1]. While exhibiting exceptional specificity, stability and solubility, these antigen-binding domains are only one-tenth in size compared to conventional antibodies [3]. All these unique features make nanobodies a promising therapeutic and imaging agent … but wait, how could we generate the nanobodies we want in the first place?
You may already have an answer by now. Yes, alpacas (though other camelid family members could also be used)! In a typical screening process [4], scientists would first immunise alpacas with different antigens to induce the production of respective antibodies by their B cells. After extraction of alpaca B cells and their mRNAs, scientists would reverse transcribe the mRNAs to synthesise double-stranded cDNAs. Then, specially designed primers are used to amplify the DNA sequences coding for the variable regions through polymerase chain reaction (PCR). Next, scientists would introduce recombinant plasmids carrying different variable region sequences to phages (a type of virus), causing them to express respective nanobodies on their surface. Phages displaying nanobodies that could bind to target antigens would then be selected, and the DNA sequences coding the nanobodies of interest can be revealed by DNA sequencing. With this piece of information, we can genetically engineer Escherichia coli bacteria to mass-produce the nanobodies we need [5].
Figuring out the distribution of cancerous tissue in a patient's body is crucial to cancer diagnosis and subsequent treatment. To image a tumour tissue by positron emission tomography (PET), a detectable radioactive tracer (probe) that specifically binds to the target tumour antigen is needed. Undoubtedly, a highly specific nanobody is an ideal probe. To detect the nanobody, scientists devised a smart solution of tagging the nanobody with radioisotopes like fluorine-18 or zirconium-89 [6]. The reason why nanobodies are preferred over conventional antibodies is because of their small sizes. Being small allows nanobodies to easily penetrate tumour tissue, thus potentially revealing a larger number of cancer cells in hiding.
Similar to conventional antibodies, nanobodies can be applied as therapeutic agents. In 2020, a group of scientists from Sweden reported an exciting discovery that an alpaca-derived nanobody could neutralise SARS-CoV-2 by blocking its interaction with a host cell receptor, hence preventing the virus from entering and infecting the host cell [7]. Nanobodies also hold promise as potential cancer therapies. Scientists have been developing nanobody-based drugs for colon, breast and liver cancer [4]. They believe these drugs could block important cancer cell signals, or act as delivery vectors of chemotherapy and radiotherapy to deliver molecular drugs or radioactive compounds to the tumour, to kill cancer cells.
Beyond disease-related applications, scientists also use nanobodies for live cell imaging. By fusing alpaca nanobody with a green fluorescent protein, researchers were able to visualise the actions of target proteins during immune response in real-time [8]. Structural biologists are fascinated by nanobodies, too. They have used nanobodies to help determine protein structures by X-ray crystallography (footnote 1) and cryo-electron microscopy (footnote 2) [9-11].
The above list is definitely not exhaustive; scientists are still actively exploring other amazing applications of nanobodies. Next time when you visit an alpaca farm with your family and friends, don't forget to share with them the wonders inside these cute creatures!
Footnotes:
- X-ray crystallography: A common technique used to find out the three-dimensional molecular and atomic arrangement of a crystallised sample. The sample is exposed to X-rays and the resulting X-ray diffraction pattern can be used to determine the sample's structure. However, preparation of crystallised sample could be challenging, and nanobodies is a tool to increase crystallization probability [10].
- Cryo-electron microscopy (cryo-EM): A method that uses frozen samples and less intense electron beams compared to traditional transmission electron microscopy, in which biomolecules may be burned or destroyed by the high energy electrons [12]. Nanobodies allow the study of small proteins (<100 kDa) by cryo-EM, which was technically challenging in the past [11].
References:
- Hamers-Casterman C, Atarhouch T, Muyldermans S, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993;363(6428):446-448. doi:10.1038/363446a0
- Muyldermans S. Single domain camel antibodies: Current status. Reviews in Molecular Biotechnology. 2001;74(4):277-302. doi:10.1016/s1389-0352(01)00021-6
- Salvador J-P, Vilaplana L, Marco M-P. Nanobody: Outstanding features for diagnostic and therapeutic applications. Analytical and Bioanalytical Chemistry. 2019;411(9):1703-1713. doi:10.1007/s00216-019-01633-4
- Sun S, Ding Z, Yang X, et al. Nanobody: A Small Antibody with Big Implications for Tumor Therapeutic Strategy. International Journal of Nanomedicine. 2021;16:2337-2356. doi:10.2147/ijn.s297631
- de Marco A. Recombinant expression of nanobodies and nanobody-derived immunoreagents. Protein Expression and Purification. 2020;172:105645. doi:10.1016/j.pep.2020.105645
- Yang EY, Shah K. Nanobodies: Next generation of cancer diagnostics and therapeutics. Frontiers in Oncology. 2020;10:1182. doi:10.3389/fonc.2020.01182
- Hanke L, Vidakovics Perez L, Sheward DJ, et al. An alpaca nanobody neutralizes SARS-COV-2 by blocking receptor interaction. Nature Communications. 2020;11(1):4420. doi:10.1038/s41467-020-18174-5
- Schmidt FI, Lu A, Chen JW, et al. A single domain antibody fragment that recognizes the adaptor ASC defines the role of ASC domains in inflammasome assembly. Journal of Experimental Medicine. 2016;213(5):771-790. doi:10.1084/jem.20151790
- Domanska K, Vanderhaegen S, Srinivasan V, et al. Atomic structure of a nanobody-trapped domain-swapped dimer of an amyloidogenic Β2-microglobulin variant. Proceedings of the National Academy of Sciences. 2011;108(4):1314-1319. doi:10.1073/pnas.1008560108
- Koide S. Engineering of recombinant crystallization chaperones. Current Opinion in Structural Biology. 2009;19(4):449-457. doi:10.1016/j.sbi.2009.04.008
- Wu X, Rapoport TA. Cryo-EM structure determination of small proteins by nanobody-binding scaffolds (Legobodies). Proceedings of the National Academy of Sciences. 2021;118(41):e2115001118. doi:10.1073/pnas.2115001118
- Broadwith P. Explainer: What is cryo-electron microscopy. Chemistry World. https://www.chemistryworld.com/news/explainer-what-is-cryo-electron-microscopy/3008091.article.
Author:
Helen Wong, Student Editor, Science Focus, The Hong Kong University of Science and Technology
March 2024

